To assess the safety and effectiveness of their Covid-19 vaccines, BioNTech SE, Pfizer Inc. and Moderna Inc. sponsored clinical trials overseen by independent boards that recruited tens of thousands of participants, randomly assigned them to receive vaccines or placebos and waited to see if there was a discernible difference in outcomes between the two groups.
With the rapid spread of the new coronavirus in the U.S. this fall, they didn’t have to wait long — and the results were stunning. In both trials, about 95% of those who developed Covid symptoms were in the placebo group, meaning that the vaccines appeared to be 95% effective.
A chart from a U.S. Food and Drug Administration briefing document illustrates that effectiveness perhaps more clearly than the percentages do. It shows the cumulative incidence of symptomatic Covid-19 cases in the days following the first dose of the BioNTech-Pfizer vaccine (in blue) versus a placebo (in red).
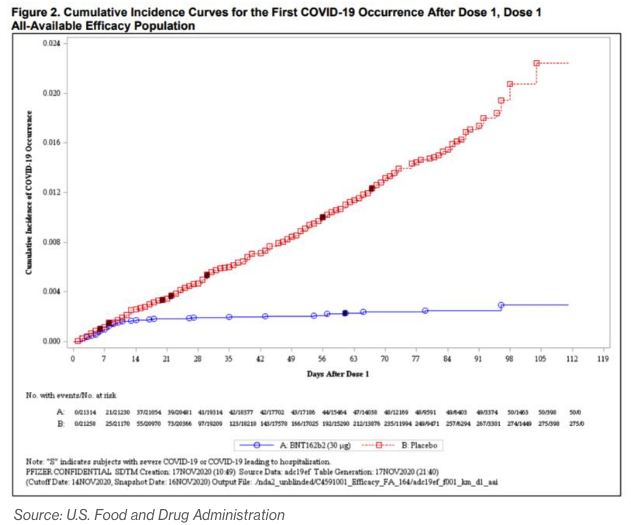
The chart for the Moderna vaccine is nearly identical. What both show is that for the first 10 days or so after getting a shot, people in the placebo and vaccine groups were infected with the virus at nearly identical rates. After that, the placebo recipients kept on getting Covid-19 at the same pace, while those who received the vaccine hardly got it at all.
These results don’t bring absolute certainty about how well the vaccines will work, or for how long, or what if any long-term side effects they might bring. But they provided convincing enough evidence to persuade the FDA to grant emergency-use approvals for both, with multiple peer agencies abroad taking similar steps, and to offer realistic hope that the pandemic could be over by summer.
If only most of the other virus-related studies delivered results like that! Consider the new mutation of SARS-CoV-2 (the virus that causes Covid-19) that was first identified in the U.K. in September and about which epidemiologists began ringing alarm bells last month. It seems to be more contagious than earlier variants — how else could it have so quickly become the country’s dominant strain? But is it 70% more contagious, as originally estimated? Or 56% more, as one quick-turnaround study concluded last month? Or something more like 10% to 20%, which some experts still hold out hope for?
We’ll probably never know for sure. There’s no plausible and ethically defensible method for comparing the contagiousness of different virus variants in the controlled way that vaccines are tested, plus the exact answer just isn’t that important. Far more crucial is whether existing vaccines can stop it. The early thinking is that they can, and we should get a lot more information on that over the next couple of months.
So it goes with the state of knowledge on Covid-19. There’s certainly a lot of it: 254,798 Covid-related articles, working papers and other publications had been produced as of Tuesday, according to the Dimensions database maintained by Digital Science & Research Solutions, Inc., with 7,871 clinical trials completed or in the works. But there are huge differences in the reliability, importance and durability of the findings.
At one end are large, randomized controlled trials such as the vaccine studies. The great strength of that RCT research method, which physicians first started dabbling with in the 1700s and developed into its modern form in the decades after World War II, is that researchers can hold more or less everything equal except for the vaccine or other treatment being tested, allowing them to draw clear connections between cause and effect.








